KoBIA (Korea Biomedicine Industry Association)
The Korea Biomedicine Industry Association (KoBIA) represents the Korean biomedicine industry, established in 2011. Our mission is to contribute to the promotion healthcare industry and the Improvement of national health by reinforcing the global competitiveness of the biopharmaceutical industry as the new national growth engine. 
Main Activities
Research on biopharmaceutical policies and systems and collaboration with the government and member companies
Gateway for communication in the biopharmaceutical industry
– Dynamic BIO – Strategic planning bureau for the development biopharmaceutical industry
– Market Access Committee
– Discussions and debates to enhance communication between private and government
Provision of biopharmaceutical industry information
– 「Click! Global Biopharmaceutical information」 website management support
– Provision biopharmaceutical industry statistics
– Publication trend reports on the biopharmaceutical industry
Promote Korean biopharmaceutical industry international cooperation and supporting global
– Global Bio Conference, GBC
– Offering support for relevant local and overseas conferences, forums, etc
Develop and train biomedical experts
– GMP, Medical product manufacturing, ICH guidelines, QbD & CQAs, etc.
Visit Website: https://www.kobia.kr/
Kate (Jeong-Min) Choi / Director
Present and Future of Cell & Gene Therapy: Opportunities and Challenges in Korea
Astrogen Inc.
Founded in 2017 in South Korea by a pediatric neurologist, Astrogen specializes in developing small molecule-based treatments for neurological diseases. Our company specializes in clinical trials, electrophysiological assessments, evaluations using animal models of neurological diseases, and real-time blood-brain barrier permeability assessments using microdialysis. This specialization gives us a strong advantage in developing new drugs for neurological disorders. We currently possess a library of over 700 candidate compounds through our technology that combines molecular modeling and preclinical evaluation techniques. Additionally, we have 44 employees, 80% of whom are in R&D, and our core management team consists of professionals with over 20 years of experience in drug development.
The company’s flagship pipeline, AST-001, is a first-in-class drug targeting core symptoms of autism spectrum disorder. Compared to other global pharmaceutical companies’ drugs that have entered phase 3 clinical trials, AST-001 boasts higher safety, efficacy, and approval potential. Additionally, it has the advantage of being applicable from the age of 2 without any DDI issues. AST-001 demonstrated excellent efficacy and safety in phase 2 clinical trials and is nearing the completion of phase 3, with statistical results expected in January next year. Furthermore, AST-001 was recently designated as an orphan drug by the Korean FDA, granting it 11 years of market exclusivity.
Astrogen also has a diverse pipeline targeting various intractable neurological diseases, including Rett syndrome (AST-004), Dravet syndrome (AST-008), Parkinson’s disease (AST-029), Parkinson’s disease/idiopathic pulmonary fibrosis (AST-030), ADHD (AST-031), obstructive sleep apnea (AST-032), glioblastoma (AST-035), and neurofibromatosis type 1 (AST-038). Notably, AST-035, which operates on a molecular glue mechanism, has demonstrated superior efficacy, safety, stability, pharmacokinetics (PK), and blood-brain barrier (BBB) permeability compared to existing drugs. AST-030 and AST-038 have been successfully out-licensed to China.
Astrogen is actively enhancing its research and development efforts and is seeking strong partnerships in the UAE to establish fruitful collaborations.
Product Profile
We developed a syrup formulation of AST-001 for the convenience of patients with Autism Spectrum Disorder(Figure 1). Patients with Autism Spectrum Disorder often exhibit heightened sensitivity to sensory stimuli such as taste, smell, color, and texture, which can significantly impact their medication adherence. Therefore, the powder formulation used in Phase 1 clinical trials was changed to a syrup formulation. After extensive formulation research, we successfully developed a syrup formulation that showed no issues in a 36-month stability test(Figure 2). This formulation, optimized with taste and aroma suitable for patients with Autism Spectrum Disorder, has been patented. The technology was transferred to Nexpharm Korea, which produced the medication for use in Phase 2 and Phase 3 clinical trials.
We developed a syrup formulation of AST-001 for the convenience of patients with Autism Spectrum Disorder(Figure 1). Patients with Autism Spectrum Disorder often exhibit heightened sensitivity to sensory stimuli such as taste, smell, color, and texture, which can significantly impact their medication adherence. Therefore, the powder formulation used in Phase 1 clinical trials was changed to a syrup formulation. After extensive formulation research, we successfully developed a syrup formulation that showed no issues in a 36-month stability test(Figure 2). This formulation, optimized with taste and aroma suitable for patients with Autism Spectrum Disorder, has been patented. The technology was transferred to Nexpharm Korea, which produced the medication for use in Phase 2 and Phase 3 clinical trials. 
Figure 1. Individually packaged AST-001 syrup used in Phase 2 and Phase 3 clinical trials for Autism Spectrum Disorder 
AST-001 directly acts on the prefrontal cortex, which is associated with controlling core symptoms of autism, regulating excitatory/inhibitory neurotransmission pathways, making it effective in treating core symptoms of autism. In social behavior improvement experiments compared to existing approved drugs Risperidone, Aripiprazole, and the Phase 3 clinical trial drug Balovaptan, only AST-001 showed improvement effects(Figure 3).
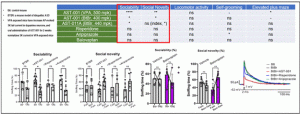
Figure 3. Experiment on the improvement of social behavior in autism model mice by Risperidone/Aripiprazole/Balovaptan/AST-001
We have internalized an optimized infrastructure for the development of treatments for neurological disorders, which includes electrophysiological evaluation, molecular modeling, and a real-time BBB permeability assessment system(Figure 4). Through this, we are continuously developing subsequent pipelines for rare diseases such as Rett syndrome, Dravet syndrome, neurofibromatosis, glioblastoma, idiopathic pulmonary fibrosis, as well as ADHD, obstructive sleep apnea, and Parkinson’s disease. 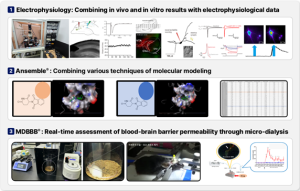
Figure 4. Continuous development of follow-up pipeline
Visit Website: www.astrogen.co.kr
Su-Kyeong Hwang / CEO
Innovative Therapies for Rare Diseases
MedySapiens, Inc.
MedySapiens is at the clinical forefront of providing testing and analysis solutions for rare diseases in newborns, with the mission and vision of “providing opportunities for a healthy life through fast and accurate diagnostic technology for rare diseases”.
MedySapiens was established in 2017 and currently has 6 teams with 17 full-time employees. We have raised about $7.7M so far and are seeking an additional $10M for series B.
Key Advantages
Through collaborative efforts, we can offer products and services to aid in diagnosing rare diseases. For the hospital, we can obtain evidence to confirm clinical significance through data and product advancement. On the other hand, we can provide interpretation reports for clinicians to make a quick and accurate diagnosis. Furthermore, through technical cooperation with pharmaceutical companies, we can provide data for the development of orphan drugs that cooperate in discovering new biomarkers and can contribute to companion diagnosis in the future. Additionally, there is potential for cooperation to attract investments and undertake national projects.
Product Profile
MedySapiens provides two main products/technologies for diagnosis & care services of newborns with rare diseases through AI-based genomics.
– Co-developed with clinical frontline MDs NEOseq_ACTION® is specialized to detect approximately 265 genes that cause over 220 rare diseases (can be customized) actionable rare diseases at once utilizing hybridization with accuracy. Also, It allows for the use of minimal sample volumes (100 µl, 1 DBS), making it suitable for NICU patients.
– MedyCVi® is an AI-driven diagnostic S/W that identifies and evaluates genetic variants associated with rare genetic diseases. It dramatically reduces the time and efforts of clinicians in checking the information of variants calling and interpretation. Utilizing a self-developed BI pipeline with integrative capabilities and scalability, along with AI modules developed for speed. The modules are used for automatic prioritization and classification of detected variants, variant interpretation through LLM-based literature analysis, and generative AI-based report customization. The WES and Targeted Sequencing are ready with MedyCVi®. Also, web or cloud-based S/W offers expandability and scalability to germline/somatic and proteomics analysis for the environment of customers. 
① Machine Leaning in the form of MedyPatho™ which offers pathogenicity scores to classify genetic variants into 3 categories, pathogenic, benign, and VUS (Variant of Uncertain Significance), or 5 categories including likely pathogenic and likely benign
② NLP (Natural Language Processing) and LLM (Large Language Model) in the form of MedyLAS™, which offer the most relevant research papers and their summaries including the relationship between genes, variants, and phenotypes out of PubMed’s 4 million papers, thousands of them being newly added each day, per association scores among genes, genetic variants, and diseases
③ Generative AI in the form of MedyGenAI™ offering genetic variant interpretation reports customized for individual hospitals’ respective needs
We outperform in the following areas:
– Optimized experimental protocol of minimized blood volume requirement, especially for sick newborns in the NICU
– Reduced TAT, turn-around time(less than 3 days)
– Improved accuracy & specificity through AI-based assessment of genetic variants
– AI solution provides clinicians with speed & genomic counselors’ level of support
– One-stop solution providing both diagnostics & care services
Visit Website: www.medysapiens.com/en
Sang Goo Kang / CEO
Diagnosis of rare diseases through AI-based genome analysis
INIBIO Co., Ltd.
INIBIO Co., Ltd. is a biopharmaceutical company specializing in the production of botulinum toxin products. Founded in December 2017, INIBIO Co., Ltd. has rapidly established itself as a leader in the biopharmaceutical industry. By receiving approval for Korea’s fifth approved botulinum toxin product from the Korean Ministry of Food and Drug Safety, INIBIO has laid a strong foundation for future growth and expansion. This achievement has enabled the company to quickly rise as a prominent manufacturer in the field, reflecting its strategic vision, ambitious goals, and exceptional execution capabilities.



We continuously develop products in various dosages to meet consumer needs and focus on new clinical developments. Additionally, we strive to create products in diverse formulations, making them more accessible and user-friendly for patients. These efforts and challenges by INIBIO are aimed at becoming a leader in the rapidly growing beauty and wellness industry. INIBIO’s journey demonstrates the power of relentless pursuit of innovation, strategic planning, and excellence.
Product Profile :
Introducing INIBO: Excellence in Botulinum Toxin
At INIBIO, our commitment to quality and innovation shines through in our flagship botulinum toxin product, INIBO. INIBO stands out in the market due to several key factors that ensure its superior performance and reliability.
Unique, Scientifically Validated Strain
INIBO is formulated using a unique strain, CCUG 7968, imported through official procedures from the Culture Collection University of Gothenburg (CCUG) in Sweden. This strain is known for its close genetic relationship to the Hall A strain used in Allergan’s Botox, having been validated by the prestigious Institut Pasteur in Paris. This exclusive strain is a testament to our dedication to leveraging scientifically validated sources for the highest quality product..
Advanced Purification Techniques
Our advanced purification technology ensures that INIBO maintains an exceptional level of purity. We use specialized columns designed to interact optimally with botulinum toxin, resulting in a formulation composed of 99.99% 900kDa protein. This high degree of purity allows INIBO to exhibit a rapid onset time, typically within three days, and minimizes unwanted diffusion during injection. These characteristics contribute to high satisfaction among both medical practitioners and patients.
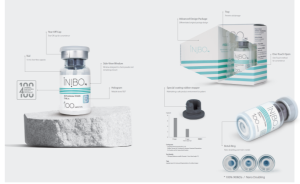
Specialized Drying Process for Superior Stability
INIBO’s stability and efficacy are further enhanced by our patented Specialized Vacuum Dry Process. This innovative technology improves product stability and reduces the presence of inactive proteins. Unlike traditional freeze-drying methods, which can cause ice nucleation and potential damage to active proteins, our vacuum-drying method preserves the integrity of the active components. This process has been patented by the Korean Intellectual Property Office and is a key factor in INIBO’s superior quality.
Commitment to Excellence
INIBIO’s dedication to producing top-tier botulinum toxin is evident in every aspect of INIBO. From the selection of our unique strain to our cutting-edge purification and drying technologies, we strive to meet the highest standards of safety and effectiveness. Our goal is to provide medical professionals with a reliable product that delivers exceptional results and to ensure patient satisfaction through innovative and effective.
Visit Website: http://www.inibio.com/
Hyeon-A Yim / CTO
Use of botulinum toxin derived from new strains as pharmaceuticals
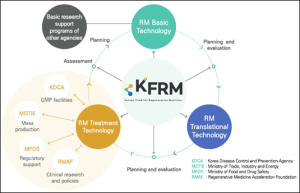
The Korean Fund for Regenerative Medicine (KFRM) was established in 2021 to address the fragmentation and redundancy in national R&D projects and to bridge the “valley of death” in regenerative medicine. Supported by Korea’s Ministry of Science and ICT and the Ministry of Health and Welfare with a budget of $430.5 million from 2021 to 2030, KFRM aims to provide comprehensive, full-cycle support for R&D in regenerative medicine. KFRM also seeks to proactively adapt to environmental changes, ensuring the advancement and practical application of regenerative medical technologies.
KFRM supports the development of advanced biopharmaceuticals, including cell therapy, gene therapy, tissue engineering, and advanced bio-composites. In 2024, KFRM is backing 149 ongoing projects. Our strategy focuses on enhancing opportunities for groundbreaking basic research, strengthening project linkages, and expanding support for commercialization and clinical trials. The program is structured into three distinct groups, each with specific goals and strategies:

KFRM’s mission is to overcome intractable diseases through the development of regenerative medicine treatments and technologies, supporting both fundamental research and clinical translation. We are dedicated to fostering innovation and facilitating the commercialization of novel medical solutions.
KFRM supports project planning, management, evaluation, and commercialization. We establish research networks, promote technology transfer, strengthen clinical translation, and analyze and utilize project outcomes.
Additionally, KFRM offers consulting services on patents, non-clinical/clinical R&D, CMC, and regulatory affairs to facilitate the commercialization of research outcomes. Our comprehensive approach ensures that projects receive the necessary support to transition from concept to market, driving advancements in regenerative medicine.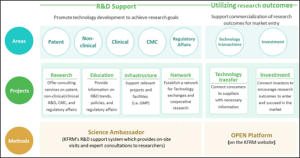
Visit Website: http://www.kfrm.org
Dr. In-Ho Jo / CEO
Current Status and Future Perspectives of Regenerative Medicine in Korea
CEFObio Co., Ltd.
CEFObio Co.,Ltd was founded to conduct research and development for cell based therapy products and commercialize them in September 2011. During the start-up period, we focused on establishing basic technologies for developing cell therapy such as 3D cell culture systems, and developing elemental technologies(culture medium, enzymes and culture wear, etc.) for cell therapy.
Since then, CEFObio Co., Ltd. developed a cell therapy by applying 3D culture technology, a secured original technology. The factory was completed in 2019 and began producing cell therapy for clinical trials in 2020. And also, it commercialized culture factors such as culture medium, enzymes, and cryosolution for cell-related therapy products by producing them in accordance with ISO13485.
In July 2021, a phase 1 clinical trial for the rare disease osteonecrosis of the femoral head(ONFH) was approved by the regulatory agency, and the phase 1 clinical trial was completed in June 2023. In addition, we are rapidly expanding the indications for spinal fusion, fractures, etc. We are currently applying for phase 2 clinical trials for fragility fractures, and non-clinical trials for spinal fusion have been completed.
In October 2023, the excellence of the cell therapy under development was proven by receiving the New Excellent Technology (NET) certification. Additionally, we are developing chondrocyte and immune cell therapies through new pipelines.
CEFObio is a cutting-edge biopharmaceutical technology development company that aims to contribute to the advancement of medical technology through know-how and technological innovation accumulated through many years of stem cell and culture optimization research and development.
Product Profile :
Key Technologies and Products
Patent : Major patents related to key technologies have been registered in Korea, USA, Japan, EU, the United Kingdom, Switzerland, Spain, Ireland, and PCT.
① Cell Therapy
CF-M801 is an early Osteoblast that induces bone regeneration and angiogenesis. It was developed by applying CEFObio’s unique 3D cell culture system to induce mesenchymal stem cells into early osteoblasts in a short period of time. And also, CEFObio has succeeded in mass-cultivating therapeutic osteoblasts so that we can provide 2,000 doses from a single master bank of allogenic cells. CF-M801 is off-the-shelf type in that it is allogenic and supplied in frozen status, comparing to the conventional cell therapy, autologous and supplied in culture flask. The initial indication is osteonecrosis of the femoral head, and the necrotic bone tissue is removed through invasive surgery and then cell therapy is implanted. We are also preparing to expand the indications to fracture and spinal diseases. CF-M801 has completed phase 1 clinical trials for ONFH and successfully demonstrated safety and exploratory efficacy. It is currently preparing for phase 2 confirmatory clinical trials.
CF-M802 is a chondrocyte cell differentiated from umbilical cord-derived mesenchymal stem cells and is being developed as a chondrocyte treatment. Natural Killer cells are being developed as an immune cell therapy and, like CF-M801, are currently in the non-clinical testing stage.
② Culture Media for cell therapy
Cell culture media is produced in accordance with ISO 13485 standards with the intention of using it in the production of advanced biopharmaceuticals. Cell culture media are developed as a Xeno-free medium and a Xenogeneic medium in the CEFOgro™ series, and the Xeno-free medium is mainly supplied to the field of advanced regenerative medicine.
③ Cell Preservative, Cell storage and delivery solution
In October 2023, the cell cryopreservation kit was approved as a medical device in Korea, and was developed with dedicated cryopreservation vials, cryoboxes, and cryopreservation agents for the cryopreservation of advanced biopharmaceuticals. In addition, a preservative agent that can stably move and store cultured cells in a non-frozen state was developed.
④ Enzymes
TExpress is a recombinant trypsin optimized to allow cell detachment without cell damage during adherent cultures and has been developed to be stored at room temperature for up to two years.
Adicol and Adicol-plus are developed as lyophilized ready-made products with collagenase as the main ingredient. The Adicol series is used for isolate cells from tissues, harvest cells during 3D culture using scaffolds, or isolate single cells from spheroids or organoids.

Visit Website: https://cefobio.com
Hyun Sook Park, CEO
Wharton’s jelly-derived cells transforming to bone and cartilage in 3D culture: Off-the-shelf cell therapy for repairing bone and cartilage defects
Cellatoz Therapeutics, Inc.
Cellatoz Therapeutics, Inc. is a biotech company with extensive expertise in translational research and process development. With our state-of-the-art commercialization-ready cGMP grade facility in Korea and unique proprietary cells, we specialize in the development of advanced and innovative cell therapies in the fields of regenerative medicine and immuno-oncology. Our flagship program, Neuronal Regeneration Promoting Cells (NRPC), involves the differentiation of Schwann cell-like cells from Tonsillar Mesenchymal Stem Cells (T-MSC) for the treatment of peripheral nerve diseases such as Charco-Marie-Tooth) disease (CMT). Further, we are developing Musculo-Skeletal Stem Cells (MSSC), which are IP-protected and possess the remarkable ability to differentiate into various components of the musculoskeletal system. These cells hold promise for regenerating cartilage and treating conditions such as sarcopenia and compound fractures. Our focus also extends to modifying the immune profile of patients to effectively treat solid tumors like glioblastoma (GBM) and recurrent ovarian cancer. We achieve this through gene modification in immune cells and combination therapy with existing drugs. Collaboration is at the core of our approach, and we work closely with major medical schools and hospitals in Korea, including Seoul National University Hospital, Samsung Medical Center, and Ewha Medical Center. We are also actively seeking partnerships in North America, Europe, and Japan to expand our reach and impact. Overall, we are committed to advancing the field of cell therapy and making a significant contribution to improving patient outcomes worldwide.
Product Profile :
Cellatoz’s proprietary cells and technologies
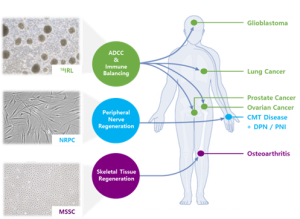
NRPC are a specialized type of cells that closely resemble Schwann cells, derived from T-MSC. Through the differentiation of T-MSC, we have successfully created a distinct and transformative cell population. These NRPC exhibit unique properties, secreting a diverse range of neurotrophic factors. These factors play a crucial role in stimulating axon sprouting and facilitating the remyelination of damaged nerves. NRPC may even integrate into the restored myelin sheaths. NRPC are a promising candidate for the treatment of various health conditions such as CMT1 disease, DPN, and PNI.
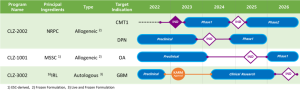
CLZ-2002 is a clinical stage program that utilizes T-MSC-derived NRPC in the treatment of CMT1 disease and was designated as an ODD by the US FDA in Feb. 2022 and by Korean MFDS in Feb. 2024. In Korea, Ph1 clinical trial IND was approved in Feb. 2023, and this IP has been finally administered to nine patients, starting with the first-line treatment in Jul. 2023. A CSR is expected around Oct. 2024. In addition, Ph1 clinical trial IND for DPN treatment applied in Korea for the purpose of expanding indications has also been approved.
MSSC are derived from pluripotent stem cells, including ESC and iPSC. These remarkable cells have the unique ability to differentiate into various cell types within the musculoskeletal system, such as bone, tendon, muscle, and cartilage. Through careful manipulation of the microenvironment surrounding MSSCs, we can guide their differentiation process. Not only MSSC are themselves proprietary, but the reagents and methodology used to produce them are also exclusive to our company. While sharing functional similarities with hSSC recently discovered by Dr. Longaker’s group at Stanford University in bone marrow, MSSC exhibit a distinct expression pattern of cell surface antigens. To fully harness their capabilities and bring benefits to patients, we actively seek collaborations with diverse partners to unlock the full potential of MSSC in musculoskeletal therapies.
CLZ-1001 is a preclinical-stage program that utilizes MSSC derived from embryonic stem cells for the treatment of KOA. Currently, GLP-toxicity studies, including tumorigenicity tests, was conducted.
Lymphocytes, a crucial component of the immune system, play a vital role by secreting cytokines and antibodies upon recognizing antigens presented by other types of leukocytes. This response allows lymphocytes to target foreign substances or pathogens. Among mature lymphocytes, there are distinct subtypes such as B cells, T cells, and NK cells, each with specific functions. NK cells can monitor and eliminate abnormal cells. They achieve this either by expressing NK activating receptors or by recognizing diminished expression of MHC Class I molecules, thus maintaining immune system balance. We have been developing 16IRL, which are autologous frozen formulations suitable for repeat dosing. Unlike traditional approaches that focus solely on activating specific cells, these 16IRL aim to rebalance the immune system. Through a proprietary high-yield manufacturing method, homogenized NK cells expressing CD16 on their surface are produced. CD16 interacts with the Fc region of IgG1, resulting in ADCC. This indicates that 16IRL have the potential to synergize with immuno-oncology drugs, enhancing their effectiveness.
CLZ-3002 is a preclinical program that utilizes autologous 16IRL differentiated from human PBMC for the treatment of GBM. After completing the preclinical studies, this program has received approval from K-ARM in March of 2023. The clinical research has begun in the early of 2024.
※ Portfolio of Lead Program
Visit Website: https://cellatozrx.com
Jae-Seung Lim / CEO & CSO
Clinical development of cell therapy for peripheral nerve and musculoskeletal system
IMMUNIQUE, INC. / MEDIPOST CO., LTD.
IMMUNIQUE is a company specialized in the development of cord blood-derived immune cell therapeutics. Our focus centers on developing regulatory T cell therapeutics to treat intractable diseases including autoimmune diseases and immunodeficiency-related diseases.
With over 24 years of experience in cord blood-related basic and clinical research, along with the commercialization expertise of our parent company, MEDIPOST, in stem cell therapeutics, IMMUNIQUE is ready to leap forward as a company specialized in immune cell therapeutics.
Product Profile:
[Polyclonal Treg Therapeutics]
IMMUNIQUE is developing universally applicable “off-the-shelf” therapeutics for various autoimmune diseases, including graft-versus-host disease (GvHD), aplastic anemia (AA), ankylosing spondylitis, and lupus.
[Gene-Edited Regulatory T Cell (CAR-Treg) Therapeutics]
Utilizing Chimeric Antigen Receptors (CAR) inserted into the surface of Treg cells, the CAR-Treg therapeutics target specific sites within organs or tissues.
IMMUNIQUE aims to develop CAR-Treg therapeutics for a range of autoimmune diseases by integrating disease-specific scFv antibody fragments within its CAR-Platform.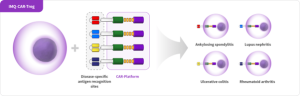
MEDIPOST (KOSDAQ 078160) is leading the global stem cell therapeutics field with the world’s first regulatory-approved allogeneic human Umbilical Cord Blood-derived Mesenchymal Stem Cell(hUCB-MSC) product named CARTISTEM® for patients with knee Osteoarthritis(OA), launched in the Korean market in 2012.
Through many years of research on characterization of cord blood-derived stem cells, MEDIPOST’s R&D Institute has been focusing on revealing the cord blood-derived stem cells’ therapeutic effects by understanding their therapeutic mechanisms. Additionally, various routes of cell administration and methods for improving cell manufacturing have been developed by MEDIPOST.
MEDIPOST is headquartered in South Korea, with its wholly-owned subsidiaries in the U.S. (MEDIPOST Inc.) and Japan (EVASTEM Co., Ltd.), as well as a stake-holding at a CDMO in Ontario (OmniaBio Inc.), Canada.
Product Profile
[CARTISTEM®]
Treatment of repetitive and/or traumatic cartilage degeneration including Degenerative Osteoarthritis(OA) without age limit.
CARTISTEM® – allogeneic umbilical cord blood-derived mesenchymal stem cells, is used for the treatment of knee cartilage defects in patients with Osteoarthritis (with ICRS grade* IV cartilage defect) caused by degeneration or repetitive trauma. To date, over 30,000 patients have been treated with CARTISTEM® in Korea with an excellent long-term safety and efficacy profile.
[PNEUMOSTEM®]
Preventive treatment of bronchopulmonary dysplasia(BPD)
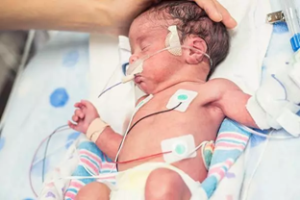
PNEUMOSTEM® an allogeneic umbilical cord blood-derived mesenchymal stem cell product, is currently under development for the preventive treatment of Bronchopulmonary Dysplasia(BPD) in premature infants. Currently, Phase 2 clinical trial is ongoing in Korea and PNEUMOSTEM® has been Orphan Drug designated by US FDA and EMA. Recently, US FDA granted a Fast Track Designation for PNEUMOSTEM®.
Visit Website: https://www.immunique.co.kr/eng
생명공학의 미래
Antonio Lee,
Global President & Executive
Tiered approach for Regenerative Medical Complex in Abu Dhabi
KIT
As a research institute under the Ministry of Science and ICT of the Republic of Korea, Korea Institute of Toxicology has secured public safety by globalization of domestic GLP toxicology tests and played the leading role in the development of biopharmaceutical industry since its foundation in 2002.
Additionally, we lead the development of source technology for next-generation toxicity assessments, expand the chemical toxicology research for the public safety, develop testing technology to support national industry innovation growth, and innovate the infrastructure to take the lead in the growth of national industry.
Deep-Tech Incubator Projects for Startups 1000+ (DIPS 1000+), run by the Ministry of SMEs and Startups and the Korea Institute of Startup & Entrepreneurship Development, is a support program aimed at discovering and nurturing promising companies with innovative technology and global expansion capabilities in the top 10 cutting-edge fields. The Korea Institute of Toxicology specializes in the bio-health (pharmaceuticals, materials) sector, providing support programs such as bio-verification, consulting, professional training, global commercialization support, mentoring, and networking. As of 2024, we have supported and nurtured approximately 150 domestic companies, achieving results in technology transfer, attracting investment, globalexpansion, and technology commercialization.
Visit Website: https://www.kitox.re.kr
YOUTH BIO GLOBAL
Management Team:
Founder & CEO : JUSTN(SEUNG HO) YOO / Chief Technology Officer: SANG MO,BAE(Plastic Surgeon/Ph.D)
Seung Ho(Justin) Yoo holds a Ph.D.(Medicine) and an MPH from Seoul National University. With a diverse career spanning various prestigious roles, including serving as an evaluator of the Advanced Regenerative Medicine Clinical Research Support Project and an Adjunct Professor at Dongguk University’s Biomedical Engineering Department since 2017. Additionally, he has been involved in multiple associations and committees related to healthcare and regulatory affairs. Dr. Yoo has received numerous awards for his contributions to the field, including recognition from government agencies and healthcare institutions. Moreover, he holds over 10 patents and has authored or co-authored over 10 papers/abstracts.
Company:
We were established in August 2017 with the purpose of providing universal medical service to patients suffering from rare and incurable diseases. We want to become a leading global healthcare provider that contributes to improving human health and quality of life based on cutting-edge diagnostic and treatment technologies.
Financing:
– CEO has about 76% of Stocks- Angel investors ONLY
Seek 1st VC investment
Use of Funds:
65% Product Development(CMO & Clinical)
11% Marketing/Sales
20% Operation/Inventory
2% Existing Debt
2% Legal/Other
Bank: KEB HANA Bank & Shinhan Bank
Investment Bank: IBK Bank
Accounting: Donghyun Accounting Firm
Attorney: SANG HEE WON(Law Office of Tae K. Song)
Patents : More than 30+ patents registered or applied.
The Business: Short-term advantages include diversification of business by expanding into various ischemic
diseases such as myocardial infarction, stroke, and retinopathy, starting with the development of vascular stem cell therapies targeting the diabetic foot indication(designated as a fast track). Our company plans to
target the total wound care and vascular stem cell market through the sale of stem cell culture medium and wound dressings based on our patented technology. Then, we plan to expand our business area and increase our market share by developing and conducting clinical trials, obtaining product approvals, and licensing out for some indications. Based on this excellent vascular stem cell technology, we expect to accelerate our growth rate by conducting R&D with high expandability, such as stem cell therapies and combination products, etc.
Financial Performance :Projections
![]()
Product Profile:
Technology: EPC(Endothelial Progenitor Cells) Priming Cell Culture Media for Vascular Stem Cells: Newly developed a cell culture medium using fully natural ingredients having anti-inflammation, anti-oxidation, and anti-virus effects has been released with both RUO(research use only) and GMP grades. The cell culture media optimize cell activation, and promote vascular stem cell activation, thereby enhancing their proliferation and mass production capacity. It also promotes the cell activation of endothelial cells and mesenchymal stem cells outside the vascular stem cells being partly collaborated with SARTORIUS Korea. It has been prominently certified and registered on the Innovation Mall and Public Procurement Service in Korea. In addition, the wound healing devices using some of those natural ingredients had registered under both MFDS Korea and US FDA with KGMP & ISO13485 certification. Vascular Stem Cell Therapeutics : This therapy utilizes allogeneic Endothelial Progenitor Cells (EPCs) derived from human umbilical cord blood, which function as stem cells(with completly animal free). Based on their differentiation ability, they exhibit excellent efficacy in regenerating blood vessels and can survive for a long time in the vessels. As they can be mass-produced in vitro, they will be commercialized as a drug product(vial & pre-filled syringe type). Their applications in treating various ischemic vascular diseases, such as diabetic foot ulcers, diabetic retinopathy, myocardial infarction, and stroke, is outstanding
Competition: The most advanced cell therapy with uni-potent, safe,
Highly effective and prominently showing both Stem Cell & Endothelial
Cell Markers(ONE & ONLY showing angiogenesis & vasculogenesis)
Visit Website: http://en.youthbioglobal.com
Seung Ho Yoo, CEO
Cutting-edge Wound care and Ischemic vascular disease treatment solutions
K-BioCELF Inc.
K-BioCELF Inc., was established in March 2019, and a Bio Venture company whose main businesses is the developments of technology based Automated Bioprocess Robotic Systems, in vitro Diagnosis, and New Drugs, based on core technology.
K-BioCELF Inc. pioneers advanced bioprocess automation through products such as the CELF™ System for advanced biopharmaceutical automatic manufacturing device and the CELB ACE™ System for in vitro diagnostic medical devices.
Major products include CELF™ (Automated Cell Culture System), CELB ACE™ System (Liquid Biopsy System, IVD), CELB™ SEP-STD (Automated Blood Separation System), CELP™ (Artificial Skin Model Production System), CELH™ DDR-SP (Reagent Dispensing System), CELH™ DST (Automated Microbial Inoculation System), and in vitro Diagnostic Kits. These products cover a wide spectrum of biotechnology and medical applications.
Partnering with government agencies, we’re developing prototypes for bioprocess automation equipment, contributing to National healthcare initiatives.
We’re scaling up production to meet the demand for biopharmaceuticals, improving accessibility and quality of life for patients worldwide.
Through our initiatives, we aim to lead the healthcare revolution and leverage automation to improve the efficiency and effectiveness of diagnosis and treatment.
Product Profile:
CELF™ (Automated Cell Culture System)
CELF™ technology consists of liquid handling, precision control, cell image processing, and centrifugal motion control technologies. It is a product that modularizes the cell culture process (sample separation → cell culture → proliferation → coating → cell collection → cell distribution → cell differentiation → cell harvest → cell banking or filling) by combining these technologies.
Composed of unit modules for each function of cell culture/proliferation, coating, cell collection, cell division, cell differentiation and cell harvesting process.
Application to Human Advanced Biopharmaceuticals and Bio food (Cultured Meat)
CELF™ BR : Gravitaxis-based monitoring Bioreactor System. It is a product developed by cell growth monitoring technology according to the change in the distribution of Gravitaxis inside the incubator : Application to R&D
Visit Website: www.k-biocelf.com
Myung-Ryurl Oh / CEO
Automatic Manufacturing System of Advanced Biopharmaceuticals
SML BIOPHARM
SML Biopharm is an innovative company leading the research and development of mRNA vaccines and therapeutics. Established in 2021 by Jae-Hwan Nam, who is a pioneer and leader in mRNA vaccine research in Korea, SML Biopharm combines a cutting-edge mRNA platform, which gained significant attention post-COVID-19, with an advanced LNP drug delivery system to develop next-generation cancer vaccines and treatments for various diseases.
SML Biopharm possesses two key technologies and patents:
Utilizing these technologies, SML Biopharm is developing pipelines for inducing immune responses to overcome cancer and other diseases, as well as pipelines for treating diseases through in vivo protein expression. Each pipeline targets a variety of diseases, with plans to expand into personalized cancer vaccines, vaccines for new infectious diseases, rare diseases, and others. Additionally, the company is working to enhance its core technologies for the development of next-generation mRNA and LNP.
Through continuous research, development, and innovation, SML Biopharm strives to achieve outstanding results in mRNA vaccine and therapeutics field and maintain competitiveness in the global market. The company is committed to growing as a global leader in mRNA-based vaccines and therapeutics, with the goal of providing hope to suffering patients and creating a better future.
Product Profile
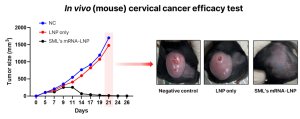
Based on these results, SML Biopharm plans to enter the preclinical stage for the cervical cancer vaccine pipeline soon. Additionally, SML Biopharm is conducting research on the next-generation personalized cancer vaccine. This approach involves analyzing the patient’s genes to identify cancer-causing neoantigens and then creating and administering mRNA-LNP containing information about these antigens to induce an anti-cancer response. In related research, SML Biopharm has confirmed effective results in vivo (mouse) studies targeting colon cancer and will continue to advance development.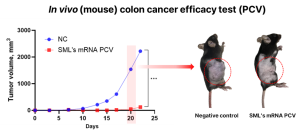
Visit Website: www.smlbiopharm.com
Yeong-Chan Ahn / R&D planning & BD, Senior Manager
mRNA based therapeutics: Innovative and personalized strategies for treatment utilizing in vivo System
Lmito Therapeutics Inc.
Lmito is an innovation-driven biotech that develops novel and effective therapies for autoimmune disease and fibrosis through an emerging concept of metabolic reprogramming.
In the immune microenvironment (IME), Lmito’s orally available small molecule compounds exert the macrophage polarizations by modulating immuno-metabolisms, with showing efficacy in the in vivo models of Inflammatory Bowel Disease (IBD), Rheumatoid Arthritis (RA), and Ankylosing Spondylitis (AS).
Lmito compounds can modulate fibro-metabolism of activated myofibroblast in the fibrosis microenvironment, resulting in anti-fibrogenic effects in both inflammatory-dependent or -independent fibrosis such as Idiopathic Pulmonary Fibrosis (IPF).
Lmito also has SARM1 inhibition program for neurological disorders which holds the potential to be a revolutionary therapy for Charcot-Marie-Tooth Disease (CMT) and Amyotrophic Lateral Sclerosis (ALS).
In the PKM2 activation program, Lmito’s orally available small molecule compounds showed in vivo efficacies of RA and IPF through blocking the nuclear translocation of dimeric PKM2 resulted in alleviating inflammation and fibrosis through metabolic reprogramming of immune cells and myofibroblasts, respectively.
Lmito has a very strong intellectual property portfolio, consisting of two material patents. Lmito is currently in the Series B funding stage and is enthusiastically raising additional funds to propel our research endeavors forward.
We have been recognized for our innovative approach and potential, as evidenced by our selection as a member of the inaugural JLABS Korea by Johnson & Johnson in the therapeutic area of immunology.
Key Highlights.
Product Profile:
Lmito Therapeutics’ core technology is based on the development of first-in-class, small molecule compounds that target cellular metabolism through an emerging concept of ‘metabolic reprogramming’ from an anabolic state to a catabolic cellular metabolism. Our compounds selectively modulate the cellular metabolism of pathologically activated cells in diverse microenvironments, such as inflammatory immune cells, myofibroblasts, and dysfunctional neuronal cells, while preserving the normal physiological functions of healthy cells.
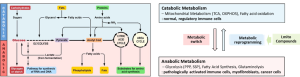
This targeted approach allows for the safe and effective treatment of autoimmune diseases, fibrotic disorders, and neurological conditions without the adverse effects associated with conventional therapies.
Our lead product candidates are orally bioavailable, new chemical entities (NCEs) that have demonstrated efficacy in preclinical models of various diseases:
Our compounds have demonstrated favorable safety profiles in preclinical studies, and we have a strong intellectual property portfolio with two granted material patents protecting our novel technology. As we advance our pipeline through clinical development, we are committed to further elucidating the mechanisms of action and therapeutic potential of our metabolic reprogramming approach in addressing the unmet needs of patients with autoimmune, fibrotic, and neurological disorders.
Visit Website: https://www.lmito.com/
Whee-Seong Lee / CEO
Lmito Therapeutics : Innovative Metabolic Reprogramming Approach for Autoimmune Disease Therapy
PAEAN Biotechnology Inc.
PAEAN Biotechnology (PAEAN) is a clinical-stage biotech company located in Korea focusing on the development and commercialization of mitochondria as therapeutic agents to fulfil unmet medical needs. PAEAN has been developing novel technologies for mitochondria production and formulation to keep the isolated mitochondria stable.
PAEAN published 6 mitochondria-related papers and has a broad IP portfolio secure its mitochondria-based technologies and products.
– 14 registered Korean patents with 10 registered overseas patents
– 19 pending Korean patents with 16 PCT application
PAEAN’s main product, MitoTherapy, is an allogeneic mitochondria derived from cultured stem cells targeting several diseases: Polymyositis/Dermatomyositis (PM/DM), Parkinson’s Disease, Hearing loss, Ophthalmic diseases, etc. First pipeline, PN-101 for PM/DM treatment received IND approval from MFDS (KFDA) in June, 2021. It marked the first allogeneic MitoTherapy product candidate in the world approved by the regulatory body. Phase 1/2a clinical study to demonstrate the safety and efficacy of PN-101 has been completed in Q4 2023 and Phase 2b IND application is scheduled to be applied in Q3 this year.
Product Profile:
MitoTherapy Products
MitoTherapy Products are novel medicinal products developed by PAEAN using mitochondria derived from cultured human stem cells. MitoTherapy’s first product, PN-101, is a groundbreaking product designed to address different severe conditions and amongst others rare diseases. Nine PM/DM patients have successfully received their initial dose during the phase 1/2a clinical study. PN-101 has proven to be a safe treatment option as none of the nine patients experienced any dose-limiting toxicity (DLT). Also, efficacy has been proven due to improvement at week 12 from baseline.
Beside PM/DM, PN-101 exhibited significant therapeutic efficacy in an animal model of Parkinson’s disease. The findings were disseminated in a publication by the Neuroscientific Academy Society in November 2023. Concurrently, the exploration of PN-101’s efficacy for other medical indications is underway in collaboration with academic professors from university hospitals.
In order to accommodate the introduction of PN-101 into both regional and global market, PAEAN has develop a frozen mitochondria which is stable for more than 3months. This clinical-grade frozen PN-101 is ready to be used in both animal and clinical studies.
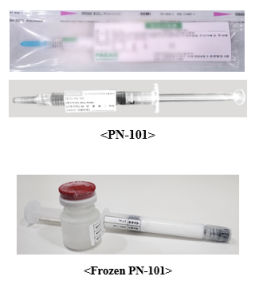
Visit Website: www.paeanbio.com
Kyu-Boem Han / CEO
Mitochondrial Transplantation/MitoTherapy
Xcell Therapeutics Inc.
Xcell Therapeutics Inc. is a leading company in the field of medium development that launched the world`s first GMP-grade next-generation chemically defined medium for human MSCs, and aims to grow into a global medium development company that meets the demands of the advanced regenerative medicine and cell-gene therapy (CGT)markets.
We are expanding our pipeline of cell-specific media targeting various cells such as MSCs, exosomes, NK, and T byutilizing “cell-specific media development source technology” (XPorT : Xcell`s Platform ; optimized media recipe forTherapeutics).
Successfully launched and commercialized the world`s first GMP-grade chemically defined medium for MSCs, CellCorCD MSC, followed the launch of CellCor SFD MSC, a serum-free medium for MSCs.
Product Profile:

Visit Website: http://xcell.co.kr/
Hyung Taek Jeon, Team leader of Department of Sales&Marketing,
Global Product Manager&BD
Key to Industrializing CGT: Game Changer to Chemically Defined Media for Next-Generation Culture Platform
CELLeBRAIN
CELLeBRAIN is a start-up company developing “Cell &Gene Therapies” to address challenging medical conditions. Our proprietary platform technologies employ mesenchymal stem cells (MSCs) engineered to express diverse therapeutic genes to treat sold cancers and chronic fibrotic diseases. Our allogeneic gene & cell therapy products are formulated as off-the shelf, frozen form to meet the high demand in the global market with substantial quantities of doses. Our therapeutic products have met the rigorous Chemistry, Manufacturing, and Controls (CMC) requirements of the Korean FDA and received clinical trial approval after demonstrating its safety and efficacy through non-clinical GLP studies.
Our therapeutic cells provide innovative modalities that combine the benefits of gene therapy and stem cell therapy, while overcoming the limitations of both. We are currently in the process of raising Series B funding and are seeking co-development partners and strategic investors for an IPO.
Product Profile:
CELLeBRAIN is a start-up company developing “Cell &Gene Therapies” to address challenging medical conditions. Our proprietary platform technologies employ mesenchymal stem cells (MSCs) engineered to express diverse therapeutic genes to treat sold cancers and chronic fibrotic diseases. Our allogeneic gene & cell therapy products are formulated as off-the shelf, frozen form to meet the high demand in the global market with substantial quantities of doses. Our therapeutic products have met the rigorous Chemistry, Manufacturing, and Controls (CMC) requirements of the Korean FDA and received clinical trial approval after demonstrating its safety and efficacy through non-clinical GLP studies.
Our therapeutic cells provide innovative modalities that combine the benefits of gene therapy and stem cell therapy, while overcoming the limitations of both. We are currently in the process of raising Series B funding and are seeking co-development partners and strategic investors for an IPO.

Visit Website: www.cellebrain.com
Hae Young Suh-Kim / CEO
Mesenchymal Stem Cells-based Gene Therapy for Solid Cancers and Fibrosis
ezCaretech
ezCaretech was established to improve patient care and innovate the medical service environment. Over the 20 years, we’ve been carrying out medical DX and IT projects in Korea and global market. And by doing so, we now have became a dominant healthcare IT company domestically and globally. Having our internal medical experts and IT professionals, ezCaretech’s performances have been proved outstanding and successful as well. Currently, we are actively running the business in the global market including Korea, Japan, U.S. and Middle East(Kingdom of Saudi Arabia and United Arab Emirates).
Product Profile
BESTCare 2.0 is the next generation HIS(Hospital Information System). This HIS is designed and developed by the current medical doctors and for that reason, it can cater various needs and requirements of medical institutes. It support and digitalize the functions of hospitals such as providing a medical treatment, managing the business and eventually increase the efficiency.
Including the medical/nursing functions and the general management, it also interfaces with a smart solution such as CDW(Clinical Data Warehouse), CLMA(Closed Loop Medication Administration), a mobile EMR, e-Consent and more to provide the most optimal work process for each member. Complying with the international standards and interfaces(HL7, DICOM, API), BESTCare 2.0 is widely used in Kingdom of Saudi Arabia, UAE, U.S and Japan.
Visit Website: https://www.ezcaretech.com/en/
Jung Sun(Charlie) Shim (Director)
HIS
Next Generation HIS(Hospital Information System) : BESTcare 2.0
With AI, we aim to make data-driven medicine the new standard of care. We are especially focused on conquering cancer, one of the leading causes of death worldwide. We develop AI solutions for precision diagnostics and therapeutics, to find the right diagnosis at the right cost, and the right treatment for the right patients.
Lunit, abbreviated from “learning unit,” is an AI software company devoted to developing advanced medical image analytics and data-driven imaging biomarkers via cutting-edge deep learning technology.
Founded in 2013, Lunit has been internationally acknowledged for its advanced, state-of-the-art technology and its application in medical images. Lunit has been named by CB Insights as one of “AI 100” startups transforming the healthcare industry and “Digital Health 150” companies.
Lunit has been chosen by World Economic Forum as one of the “Technology Pioneers” that shape the future.
Through our unprecedented AI technology, we seek to provide AI solutions that open a new era of data-driven precision medicine. Through AI solutions for diagnostic and therapeutic biomarkers, we aim to solve the most critical issues in cancer care today: reduce medical costs and prolong survival.
Lunit’s technology has been recognized at international competitions such as ImageNet (5th place, 2015), TUPAC 2016 (1st place), and Camelyon 2017 (1st place), surpassing top companies like Google, IBM, and Microsoft.
As a medical AI company, Lunit values in building clinical evidence through publishing our studies in major peer-reviewed journals. Our findings in AI detection on chest x-ray and mammography are published in Radiology, Lancet Digital Health, JAMA Network Open, Clinical Infectious Diseases, and so on.
Product Profile
Lunit INSIGHT CXR is a software device that assists interpreting physicians in the interpretation of chest radiography images. The device has been designed to automatically analyze chest radiographs via deep learning technology. The device identifies suspicious areas for abnormal radiologic findings including atelectasis, calcification, cardiomegaly, consolidation, fibrosis, mediastinal widening, nodule, pneumothorax, pleural effusion and pneumoperitoneum on chest
radiograph with 97-99% accuracy.
Validated by studies published in Radiology, European Respiratory Journal, JAMA Network Open, Lunit INSIGHT CXR can improve your reading performance, especially for critical and urgent findings such as nodule, pneumoperitoneum, pneumothorax, consolidation.
Relying on its proven accuracy, you can triage normal findings and focus on abnormalities, ultimately addressing excessive workload and shortage of radiologists in chest x-ray interpretation. Lunit INSIGHT MMG is a software device that assists interpreting physicians in the interpretation of mammograms. The device has been designed to automatically analyze digital mammograms via deep learning technology.
The device identifies and classifies suspicious areas for breast cancer on mammograms with 96% accuracy to be viewed by interpreting physicians. As an analysis result, the device allows visualization and quantitative estimation of the likelihood of the presence of a malignant lesion.
Validated by studies published in the Lancet Digital Health, JAMA Oncology, and many more, Lunit INSIGHT MMG can improve your diagnostic performance especially for dense breast and early breast cancers.
Based on its proven accuracy, you can reduce false-negative cases and recall rates, which ultimately can address shortage of breast specialists in a single-and double-reading environment.
Visit Website: www.lunit.io/en
Jae Min Oh (Vice President)
AI
Aimmed Corp.
Aimmed Co., Ltd. is a leading developer of diverse digital healthcare systems. Our innovative approach involves Digital Therapeutics, utilizing digital technology to effectively address complex mental disorders, promoting improved well-being and happiness.
Product Profile
Visit Website: www.aimmed.co.kr
Jin Hwan Lim
(CEO)
DTx
Neurophet AI
Neurophet has specialized in developing solutions for diagnosis support, treatment guides, and treatment devices targeting brain diseases based on cutting-edge artificial intelligence (AI) technology. The company was founded in 2016 by Jake Junkil Been, CEO, and Donghyeon Kim, CTO, who developed the next-generation neuro-navigation system.
Major products include brain MRI imaging analysis and interpretation software “Neurophet AQUA”, brain PET scan analysis (PET tracer deposition) software “Neurophet SCALE PET”, brain electric and magnetic stimulation simulation software “Neurophet tES/TMS Lab”, and a brain region of interest analysis research tool “Neurophet SegPlus”.
Neurophet has set its top priority to helping patients suffering from brain diseases. Based on expertise in neuroscience, Neurophet will continue to challenge and grow to explore the human brain’s health and pioneer solutions for brain diseases with AI technology.
Product Profile
Visit Website: www.neurophet.com
Young Joon Moon(CBO)
AI
AIRS Medical
AIRS Medical Co. is a Korean medical AI startup company which aims to make change in the existing inefficient healthcare industry through digital transformation of medical experience. Founded in 2018 by five co-founders from Seoul National University, the company now consists of around 100 talents and succeeded in attracting 30 billion won in Series B investment last year. Our first product SwiftMR is currently in more than 5 countries including Korea and USA with more than 250 install bases all over the world. AIRS Medical is planning to expand market coverage to 10 more countries in 2023.
Product Profile
SwiftMR is a standalone medical software that helps reduce the scan time of MRI by enhancing the quality of MRI images using elaborate deep learning AI technology. SwiftMR allows hospitals to maximize their productivity and sales by reducing MRI scan time by up to 50%. Not only SwiftMR gives benefits to medical practices, but it also helps improve MRI scan experience of patients’ to increase satisfaction for all. SwiftMR is used by more than 250 customers all over the world and is registered in more than 10 countries including Korea, US FDA, and UAE.
Visit Website: www.airsmed.com/swiftmr
Jung Joong Hwan (Head of EMEA)
AI
Heuron Co., Ltd.
Heuron is a healthcare startup that focuses on utilizing artificial intelligence (AI) and advanced image analysis to enhance the diagnosis and treatment of critical medical conditions, specifically in the fields of stroke care and neurodegenerative diseases. Heuron’s mission is to safeguard the most vital aspect of human dignity, “brain health,” using artificial intelligence. The company aims to provide essential clinical solutions needed in emergency medical settings and achieve optimal treatment outcomes for the growing number of patients with degenerative brain disorders in an aging society.
The company was established in 2017 by Dr. Donghoon Shin, a neurologist at Gachon University Gil Medical Center. It embarked on its journey with the groundbreaking ‘Heuron IPD,’ which stands as the world’s first MRI-based diagnostic AI software for Parkinson’s disease.Since then, the company has expanded its efforts, developing more than 20 diverse product pipelines.
Product Profile
Accurate and rapid diagnosis, combined with timely treatment, holds the promise of enhancing patient care for various neurological disorders while simultaneously reducing costs and risks. This objective is a shared pursuit for both medical systems and patients alike. Heuron’s AI-powered diagnostic solutions, which include the specialized Heuron AgingCare Suite™ designed for degenerative conditions such as Parkinson’s disease and dementia, as well as the Heuron StroCare Suite™ providing non-contrast CT-based stroke diagnosis, play a pivotal role in addressing this crucial challenge.
Heuron AgingCare Suite™ comprises solutions tailored to neurodegenerative diseases, including Parkinson’s disease (Heuron IPD and Heuron NI), and Alzheimer’s disease (Heuron AD and Heuron Brain PET). Leveraging MRI-based technology, this suite delivers both visual and quantitative insights, aiding in the early identification of neurodegenerative diseases and the formulation of initial treatment strategies.
Heuron StroCare Suite™ is an integrated AI solution that facilitates comprehensive patient classification for intracranial hemorrhage and cerebral infarction using non-contrast CT scans. Operating without the need for contrast agents, it analyzes and identifies emergent stroke cases, ranging from cerebral hemorrhage to large vessel occlusion, while also calculating the ASPECT score. This ensures rapid medical decision-making for patients requiring urgent interventions to minimize treatment duration.”
Visit Website: www.iheuron.com
Myungjin Shin (COO)
AI
Samsung Medical Center
Samsung Medical Center (SMC) is one of Korea’s most renowned hospitals committed to excellence in providing the highest quality, compassionate care and service to patients with 2,000 beds, 1,400 physicians, and 3,100 nurses. Since its opening in 1991, it has been leading the innovation of the healthcare sector by advocating a high-tech intelligent hospital, and now it has the highest grade in three areas(INFRAM, DIAM, EMARM) of HIMSS as the best smart hospital in Korea.
Product Profile
– Medical Support Robot : Providing remote rounds and education through AI robot in the ward
– Social Disinfection Robot : Patient guidance and multiple dense areas disinfection
– Specimen Delivery Robot : Small logistics automation system for hospital
– Logistics Robot : Automated overnight delivery system using AGV robot
Visit Website: www.samsunghospital.com/english
Jean Hyoung Lee (Assistant Cheif)
AI, HIS,Robot
ADD ABLE Co., Ltd.
Addable is a domestic respiratory medical device startup that provides data-based the customized respiratory rehabilitation solutions.
In the field of rehabilitation and medical care, we solve social problems together and seek growth together, developing products under the slogan of “Being Healthier.”
The respiratory rehabilitation solution developed by the Addable Researcher connects the breathing training device and mobile to bring about health and immunity improvement due to increased lung capacity through breathing. It is effective in reducing dementia and improving health in elderly dementia patients.
The reliability of these products is ensured by the collaboration of physical therapy, occupational therapy, elderly care experts and professors, and IT companies and designers to plan and manufacture products.
With the motif of “making life healthier”, we are trying to make products that anyone can use. The basis of life is breathing.
Addable will change the paradigm of breathing training by constantly researching and improving the Smart Breathe series.
Product Profile
Our respiratory training system is divided into two types: motorized functional recovery devices that connect a respiratory measurement device to a mobile device and are used through a dedicated application, and manual functional recovery devices that can be used in daily life while performing aerobic exercises such as running.
According to the user’s breathing volume and condition, the air volume is adjusted through the load control device mounted on the device, and the principle of breathing exercises by placing the mouth on the mouthpiece enables spirometry and respiratory muscle measurement, and reports on related breathing data are provided, and unlike the existing analog training methods (blowing balloons, blowing out candles), it is possible to continuously train by inducing user interest through games.
Our respiratory trainer products have received domestic medical device certification (class 1, class 2) and innovative product designation.
Currently, we are developing and sophisticating lung disease diagnosis and customized respiratory rehabilitation solutions using artificial intelligence technology.
Visit Website: www.smartbreathe.co.kr/en
Hyeon Seon HWA (Senior-Staff)
AI,
m-Health
People And Technology
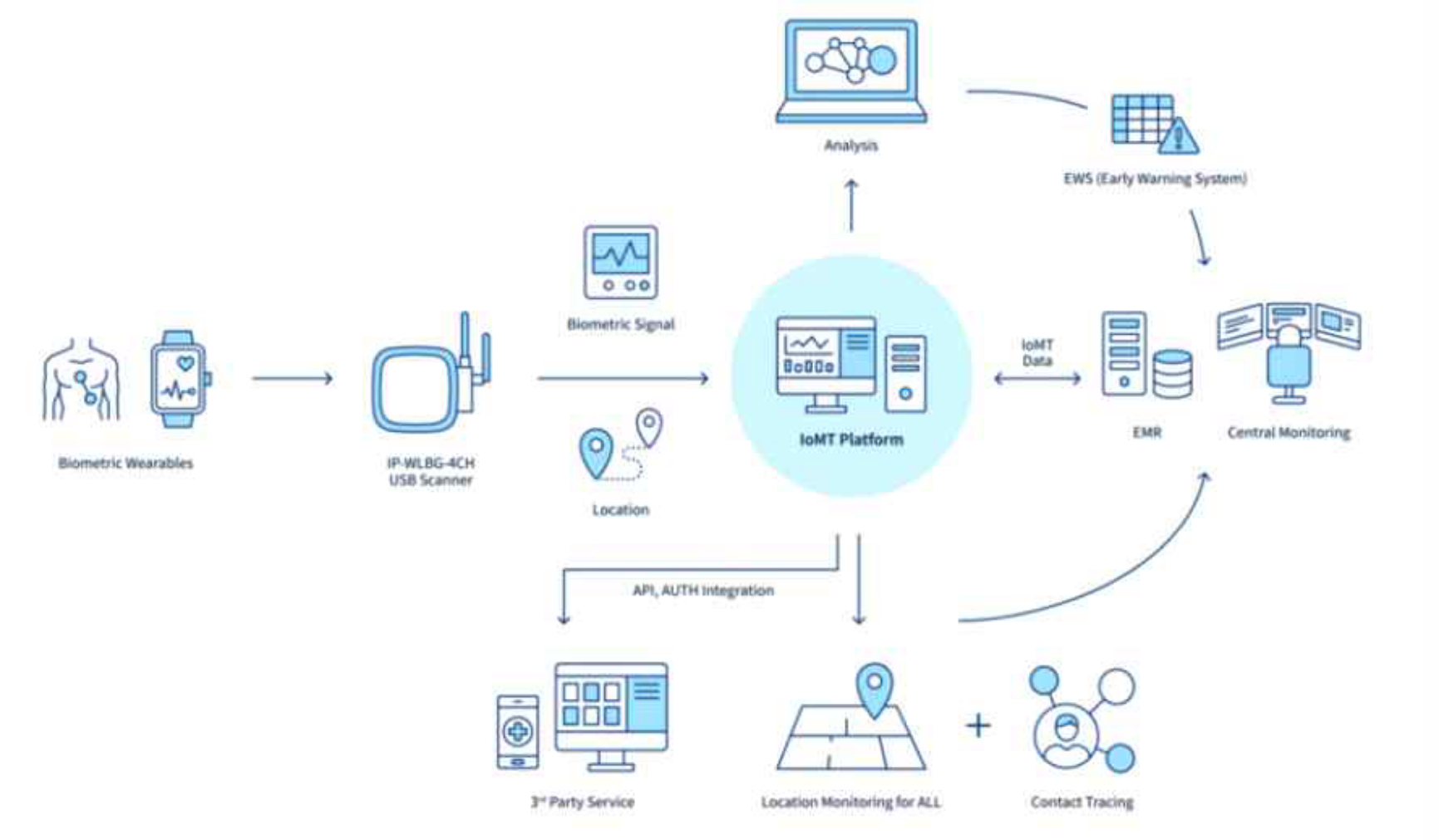
People and Technology collects, connects, and monitors various data generated in the medical hospital field, such as location information of patients, medical staff, and biometric information from medical devices, We develop IndoorPlus+ Smart Care is the solution related to efficiency, automation, and digitalization of hospital operation. We are a medical healthcare DX (Digital
Transformation) specialized solution company that develops and supplies this IoMT solution. Since its establishment in 2013, we have been developing and supplying solutions for various industries in the fields of hospitals, factories, buildings, and currently have more than 100 customers in both local and global sites.
In particular, in the hospital digitization sector, starting with the smart hospital leading model project in 2020, we have secured a track record of supplying and operating solutions to about 35 medium and large hospitals of Korea. As a result, we have been recognized as the best smart hospitals, IoMT (Internet of Medical Things) platform company.
In 2018, we established an overseas subsidary (People and Technology AG) and actively focused overseas expansion, having about 10 customer references in the Middle East and Europe. Through active business activities in the Middle East, including the KSA, UAE, we achieved overseas sales of approximately 324K USD in 2022, and are aiming for more than 100% growth in 2023.
As an result of such efforts to enter the global market, we were selected as a “notable vendor” in the IoT/IoMT field by Gartner for 2020 consecutive years in 2019, proving the excellence of the solution.
Product Profile
The IndoorPlus+ SmartCare solution is an integrated IoMT (Internet of Medical Things) platform that utilizes collected location positioning and biometric data through BLE signal collection devices to enhance the protection, operation, and management of medical assets and individuals (patients, staff, visitors) more effectively.Through the IoMT platform, various manual tasks within healthcare institutions are automated, digitized, and transformed into efficient services. By connecting diverse data generated at hospital sites, the solution addresses unmet needs in the healthcare field and ultimately provides a Smart Hospital solution that aims to enhance medical quality.
Typical IndoorPlus+ Use Cases
Key Benefits
Visit Website: www.pntbiz.com
Jean-Luc Gianduzzo (EVP)
AI,
m-Health
WAYCEN
Waycen, a company specializing in AI MEDTECH
Waycen founded by a team of eminent clinicians and AI experts with the mission of creating the next
generation of AI-enabled applications for medical and healthcare industries.
Waycen is developing and researching AI technologies necessary for the medical environment based on realtime AI technologies.
Our vision aims to be an full-cycle precision medical platform through our cutting-edged AI technology. It was recognized its competitiveness by 4 Innovation Award at CES 2023, the world’s largest IT
exhibition.
Product Profile
[WAYMED Endo]
WAYMED Endo is World-class AI SW for analyze endoscopy video in realtime during endoscopy examination.
It is compatible with all existing endoscopy suites on the market with very simple installation. so that any hospital can use our product with no limited environment factors.
It would be second observer in endoscopy room. It would help to overcome doctor’s fatigue or shorten experience.
It is recognized as a Breakthrough medical device in South Korea and CES Awarded product.
Visit Website: www.en.waycen.com
Kenny Nam (Head of Global Sales)
AI